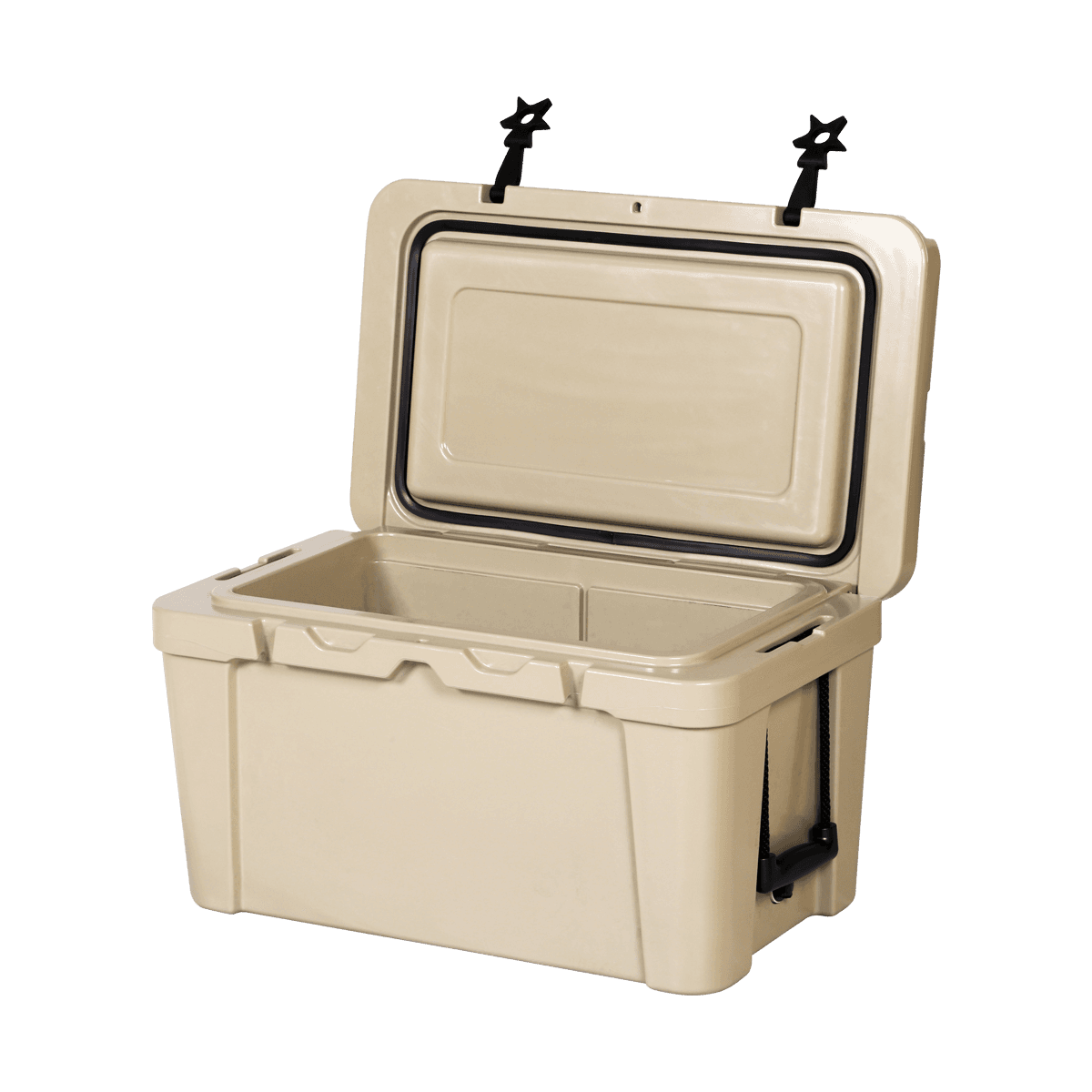
The main test methods and standards involved in the thermal insulation performance test of cold chain incubators (live fish transportation containers) include ASTM D3103 "Standard Test Methods for Thermal Insulation Performance of Transport Packages", ISTA 7D "Transportation Packaging Temperature Test", ISTA 7E "Packages" "Temperature Test of Transport Packaging in Transportation Logistics", "Quality Management Specification for Pharmaceutical Business", "General Specification for Pharmaceutical Cold Chain Incubator", enterprise standard.
At present, many developed countries have basically formed a complete cold chain logistics system. We can refer to the experience and practices of developed countries in cold chain logistics to learn and establish a cold chain logistics system suitable for our country.
In terms of the thermal insulation performance test of cold chain incubators, there is no clear specific test method and standard in our country. The current demand of chain packaging boxes can be converted into my country's national standard or industry standard, which can improve the standardization level of the insulation performance test method of cold chain incubators in my country, and has a certain application value.
As a world-renowned organization for safety testing of transport packaging, ISTA published ISTA 5B "Special Control (Refrigeration Conditions, Cold Chain) Environmental Performance Test" in 2002, which has been approved by ISTA 7D "Transportation Packaging Temperature Test" and ISTA 7E "Package Transport" It has become a method standard with specific transportation ambient temperature conditions and test requirements. It has certain applicability in the United States, but the temperature conditions cannot meet the actual logistics conditions in my country.
For some incubator packages exported to the United States, these tests are required to pass at the buyer's request. At present, the main standards in my country are the "Quality Management Practice for Pharmaceuticals" (referred to as GSP) and the "General Specification for Drug Cold Chain Incubators" promulgated by the National Health and Family Planning Commission in June 2013, as well as some enterprises' own standards, such as Q/ GYWL-01001-2013 "Enterprise Standard of Sinopharm Pharmaceutical Logistics Co., Ltd. - Verification Standard for Cold Storage, Refrigerated Truck and Refrigerated Packaging Box".
To learn more, see: Custom Insulated Storage Containers Company



-4.png?imageView2/2/format/jp2)
-4.png?imageView2/2/format/jp2)
-2.png?imageView2/2/format/jp2)

-2.png?imageView2/2/format/jp2)
-2.png?imageView2/2/format/jp2)